The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole.
Experimental measurements have determined the number of entities composing 1 mole of substance to be 6.022 × 1023, a quantity called Avogadro's number. The mass in grams of 1 mole of substance is its molar mass. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass and molar mass (g/mol) for any substance are numerically equivalent . The relationships between formula mass, the mole, and Avogadro's number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample.
Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance's mass. Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
The identity of a substance is defined not only by the types of atoms or ions it contains, but by the quantity of each type of atom or ion. For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, because a hydrogen peroxide molecule contains two oxygen atoms, as opposed to the water molecule, which has only one, the two substances exhibit very different properties.
This experimental approach required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science. To calculate this, we first need to know how many moles of nitrogen gas are in 1 kg. For that conversion, we use the molar mass of nitrogen gas, which is 28.0 grams per mole. So, 1kg of nitrogen times 1,000 grams per 1 kg times 1 mole of nitrogen per 28 grams of nitrogen equals 35.7 moles of nitrogen.
Silber4 3.140b.EOCP. Various nitrogen oxides, as well as oxides of sulfur, contribute to acidic rainfall through complex reaction sequences. Atmospheric nitrogen and oxygen combine to form nitrogen monoxide gas, which reacts with more oxygen to form nitrogen dioxide gas. In contact with water vapor, nitrogen dioxide forms aqueous nitric acid and more nitrogen monoxide. Silber4 3.140a.EOCP. Various nitrogen oxides, as well as oxides of sulfur, contribute to acidic rainfall through complex reaction sequences.
How To Calculate The Number Of Moles Of A Gas Silber4 3.070b.EOCP. Lead can be prepared from galena [lead sulfide] by first roasting the galena in oxygen gas to form lead oxide and sulfur dioxide. Heating the metal oxide with more galena forms the molten metal and more sulfur dioxide. Silber4 3.070a.EOCP. Lead can be prepared from galena [lead sulfide] by first roasting the galena in oxygen gas to form lead oxide and sulfur dioxide. To balance this reaction, we need 1 molecule of nitrogen gas, 3 molecules of hydrogen gas, and 2 molecules of ammonia. Now we have 2 nitrogen atoms and 6 hydrogen atoms on both sides of the equation.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom in the formula by the formula weight and multiplying by 100. Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) . Liquid disilicon hexachloride reacts with water to form solid silicon dioxide, hydrogen chloride gas, and hydrogen gas.
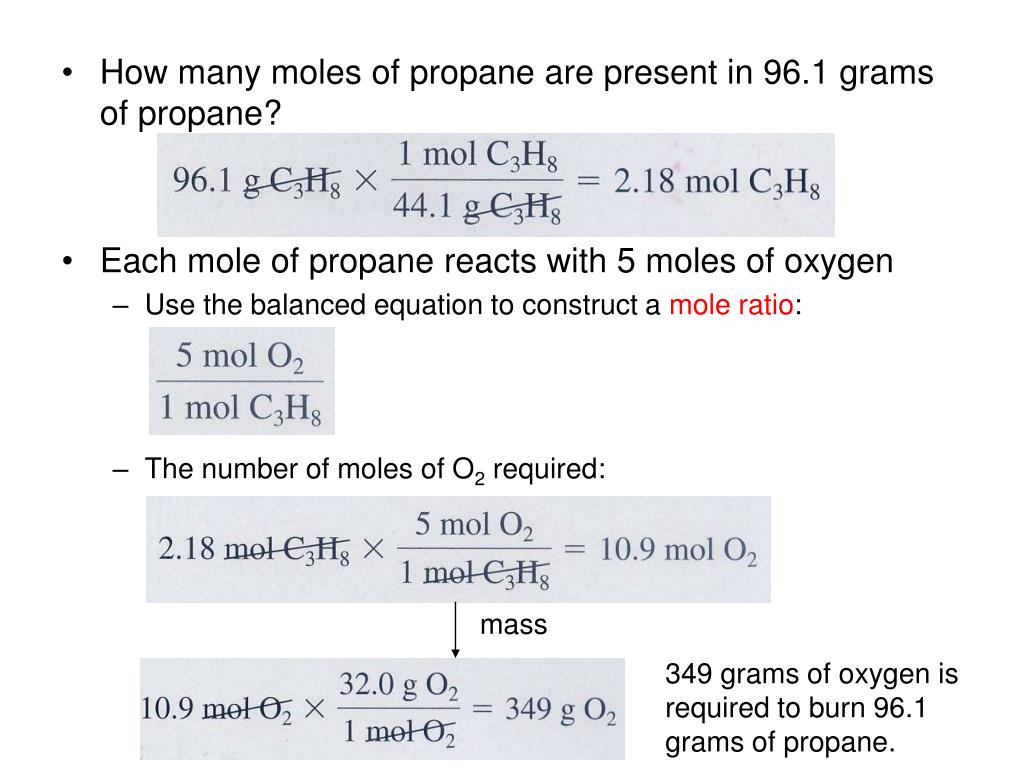
Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams. We can argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For example, Dalton's atomic theory was an attempt to explain the results of measurements that allowed him to calculate the relative masses of elements combined in various compounds. Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances.
Let's say we wanted to make 10 moles of ammonia- how many moles of nitrogen and hydrogen would we need? With our mole ratios, this is a simple problem. 10 moles of ammonia times 3 moles of hydrogen gas over 2 moles of ammonia equals 15 moles of hydrogen gas.
We can then rearrange this to get the actual mole ratio 1 mole of nitrogen gas over 3 moles of hydrogen gas is equal to 1. For the formation of ammonia, one mole of nitrogen gas is stoichiometrically equivalent to 3 moles of hydrogen gas. Another way of phrasing this is that 1 mole of nitrogen is stoichiometrically equivalent to 3 moles of hydrogen when making ammonia. This means that 1 mole of nitrogen gas will make you the same amount of ammonia as 3 moles of hydrogen.
So even though we have different amounts of each substance, when it comes to making ammonia, we can think of them as being equivalent. Said another way, to make 2 molecules of ammonia, we need 1 molecule of nitrogen and 3 molecules of hydrogen. To have a balanced reaction with no leftover atoms, the chemistry demands this ratio of molecules. The equation shows that 1 mol of sulfur reacts with 1 mol of oxygen molecules to make 1 mol of sulfur dioxide.
This means that 0.5 mol of sulfur makes 0.5 mol of sulfur dioxide. In order to figure out how many moles of nitrogen gas would contain that many molecules, you need to know how many molecules of a substance are needed in order to make one mole. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology.
This is how to calculate molar mass , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
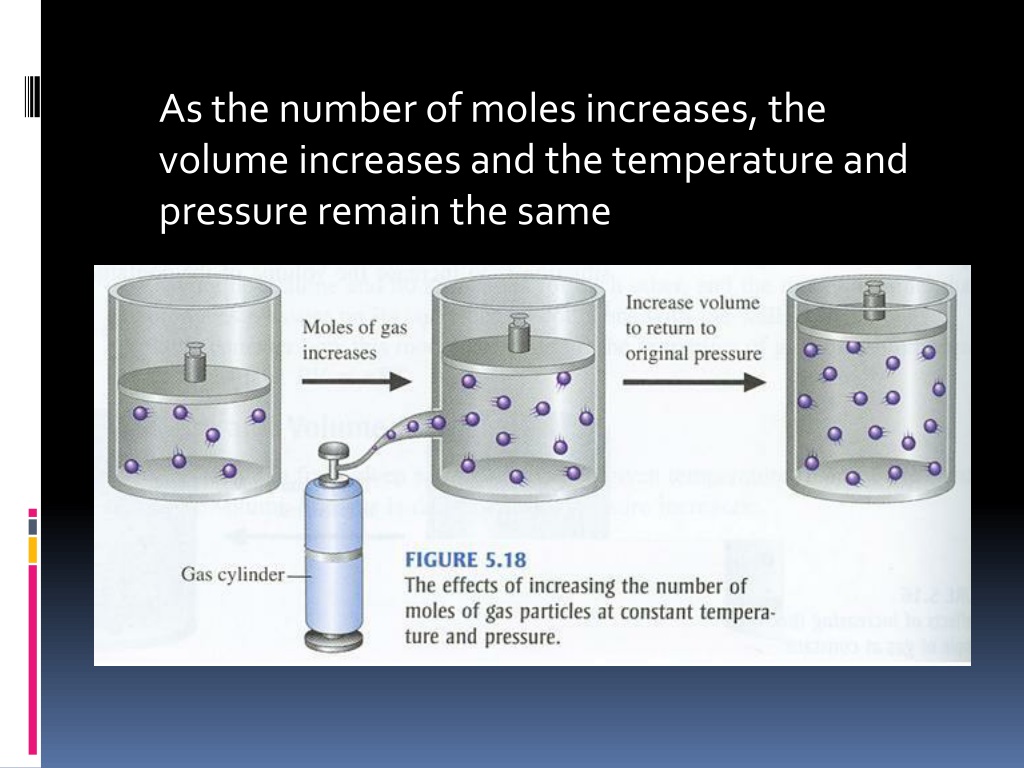
Some of the reactants also combine with oxygen in air to form zinc oxide and sulfur dioxide. When 85.2 g of Zn reacts with 52.4 g of S8, 105.4 g of ZnS forms. The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities as the number of atoms in a sample of pure 12C weighing exactly 12 g. One Latin connotation for the word "mole" is "large mass" or "bulk," which is consistent with its use as the name for this unit.
The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth. In an earlier chapter, we described the development of the atomic mass unit, the concept of average atomic masses, and the use of chemical formulas to represent the elemental makeup of substances. These ideas can be extended to calculate the formula mass of a substance by summing the average atomic masses of all the atoms represented in the substance's formula. First we will need to convert from mass into a unit that represents a count, that is, the number of particles. The mole is an accessible unit, since the periodic table provides us conversion factors in the form of atomic masses.
We can then use Avogadro's number to convert from moles to number of molecules. One mole is equal to the amount of substance that contains as many elementary units as there are atoms in 0.012 kg of carbon–12. The elementary units may be atoms, molecules, ions, radicals, electrons, etc., and must be specified. That gives us 88.2 grams of hydrogen needed to make 500 grams of ammonia.
10 moles of ammonia times 1 mole of nitrogen gas over 2 moles of ammonia equals 5 moles of nitrogen gas. Hi, and welcome to this video on the mole ratio, sometimes referred to as the molar ratio. It's a fundamental concept in chemistry that's used to convert back and forth between moles of each substance in a reaction.
Among other things, it's necessary for calculating theoretical yields and limiting reagents. This is most certainly a concept best learned through examples, so let's jump right in. For example, the equation below shows that one mole of zinc reacts with two moles of hydrochloric acid to make one mole of zinc chloride and one mole of hydrogen molecules. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Based on the formula above, we created the Grams to Moles of Nitrogen Converter below.
Please enter the number of grams that you want to convert to moles of nitrogen. The mixture of gases is then transferred into another container which has a volume two-thirds that of the original container, the temperature remaining unchanged. How many molecules are present under these new conditions?
16.8 g of calcium nitrate and 17.50 g of ammonium fluoride react completely to form calcium fluoride, dinitrogen monoxide, and water vapor. When nitrogen dioxide is bubbled into water, a solution of nitric acid forms and gaseous nitrogen monoxide is released. Write a sentence that describes how to determine the number of moles of a compound in a known mass of the compound if we know its molecular formula. The mass of K is provided, and the corresponding amount of K in moles is requested.
Referring to the periodic table, the atomic mass of K is 39.10 amu, and so its molar mass is 39.10 g/mol. The given mass of K (4.7 g) is a bit more than one-tenth the molar mass (39.10 g), so a reasonable "ballpark" estimate of the number of moles would be slightly greater than 0.1 mol. To convert moles of propane to moles of carbon dioxide, you need to use their mole ratio. In this case, for every mole of propane, you produce 3 moles of carbon dioxide. Now that we have the moles of nitrogen, we must convert to moles of ammonia.
35.7 moles of nitrogen times 2 moles of ammonia over 1 mole of nitrogen equals 71.4 moles of ammonia. Of course, we could flip the ratio if we needed to convert from moles of nitrogen to moles of hydrogen. We now can use this to convert between moles of hydrogen to moles of nitrogen, which will be very useful when we need to calculate yields or limiting reagents. Use the equation below to calculate the amount in moles of oxygen molecules that reacts with 2 mol of magnesium metal. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert.
The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass. Moist Air - Mole Fraction of Water Vapor - Mole fraction of water vapor is the ratio of water molecules to air and water molecules. At the same temperature and pressure equal volumes of all gasses contain the same number of molecules.
When hydrogen gas is passed over powdered iron oxide, iron metal and water vapor form. 62.5 g of aluminum nitrite and 54.6 g of ammonium chloride react completely to form aluminum chloride, nitrogen, and water. Determine limitiing reactant by choosing one reactant and determining the amount of the other one that is needed to completely react. Get the mole ratios from the balanced equation. Its formula has twice as many oxygen atoms as the other two compounds . Therefore, 0.60 mol of formic acid would be equivalent to 1.20 mol of a compound containing a single oxygen atom.
A typical human brain weighs about 1.5 kg and occupies a volume of roughly 1.1 L. One neurotransmitter that has been very extensively studied is dopamine, C8H11NO2. Dopamine is involved in various neurological processes that impact a wide variety of human behaviors. Dysfunctions in the dopamine systems of the brain underlie serious neurological diseases such as Parkinson's and schizophrenia.
And lastly, we convert the moles of ammonia to kilograms of ammonia with its molar mass, which is 17.0 grams per mole. So, 71.4 moles of ammonia times 17 grams of ammonia over 1 mole of ammonia times 1 kg over 1,000 grams equals 1.21 kg of ammonia. So, to make 10 moles of ammonia, we need 15 moles of hydrogen and 5 moles of nitrogen. The equation shows that 2 mol of magnesium metal reacts with 1 mol of oxygen molecules. Therefore, the mass of 2 moles of nitrogen atoms is 28g.
Which shows that for each mole of reactant, two moles in total of products are formed. 2 Each gas is undergoing an expansion when introduced into the 10.00 L container, so each will exert a partial pressure which is less than its original pressure. As temperature and number of moles of each gas in unchanged, Boyle's Law can be used to calculate the new pressure of each gas in the mixture.
When crystalline potassium chlorate is heated to just above its melting point, it reacts to form two different crystalline compounds, potassium chloride and potassium perchlorate. When solutions of calcium chloride and sodium phosphate are mixed, solid calcium phosphate forms and sodium chloride remains in solution. Get the mole ratios from the balanced chemical equation.
Explain.AnswerIonic compounds do not have molecules. There is no single unit that retains the properties of a compound. Define a molecule two different ways.AnswerA molecule is two or more atoms that are hooked together, are stuck together, and act as one unit. A molecule is the smallest piece of a substance that retains the properties of that substance.